VIEWS ON SOURCE OF ARSENIC POISONING
Experts like Kinniburgh, (BGS, U. K.) relate the presence of excess arsenic in groundwater to the geological formation of Bangladesh. Some, however, differ: they suggest that the origin of arsenic-rich groundwater is human-made. It is related to the rate of groundwater extraction. Their hypothesis, called the pyrite oxidation thesis, describes how arsenic gets mobilised in the groundwater
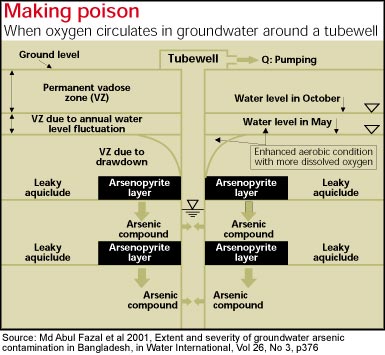
In the pyrite oxidation hypothesis, arsenic is assumed to be present in certain minerals (pyrites, iron containing rocks) that are deposited within the aquifer sediments. Due to the lowering of the water table below deposits, arseno-pyrite - oxidised in a zone of the aquifer called the Vadose zone - releases arsenic as arsenic adsorbed on iron hydroxide. During the subsequent recharge period, iron hydroxide releases arsenic into groundwater
Aerobic Hypotheses:
Aerobic Hypothesis By the Indian Scientists
The "Seed-Fertiliser-Water" technology popularly known as "Green Revolution" was recognised by the governments of this Sub-Continent in the early 1960s in maintaining food-population balance. Before 'Green Revolution' farmers rarely used modern chemical fertilisers, pesticides and irrigation. India has the largest irrigation system in the world over 70 million hectares under irrigation (Vergehse, 1990). The World Bank is one of the principal promoters of groundwater irrigation. Some experts presume that the excessive groundwater extraction for irrigation has leaked out the underground layer of arsenic. D. Chakroborti (1995) of Jadevpur University; W. Bengal, India says:
Overpumping has lowered the water table in the rocks. As the water level has fallen, the arsenic bearing sulphide rocks dry out. Oxygen penetrates the rocks, oxidising the sulphur minerals. This in turned freed arsenic to be dissolved in the groundwater , and washed into the wells.
The arsenopyrite could weather under aerobic conditions (pumping and lowering groundwater table) and release sulphuric acid and arsenic The dissolution of 1 mol arsenopyrite produces 2 moles of sulphate and arsenic in groundwater. If there are no aqueous sulphate present, it can precipitate as gypsum but gypsum is very soluble mineral and requires high concentrations of both Ca+2 and S04 -2. Other potential sinks for sulphate are melanterite (Fe (II) S04. 7H20 and diarist (K Fe3 (III)(S04) 2(OH)6, which generally occurs with acid mine drainage. Sulphate reduction is catalysed by a variety of organisms, including higher plants, algae, fungi and bacteria. Anaerobic bacteria (Desulfovrio, desulfotomaculum) can also reduce it. Sulphidogenesis occurs only under extremely reducing conditions (Eh below -250 mV).
But no such arsenic rich or arsenopyrite rich layers found in Bangladesh. The Holocene sediments in the Bay of Bengal well protected by rythmatic sedimentation containing primary sediments show any such concentration (Anwar, 2000).
Genesis of Arsenic in Ground Water Delta
A recent study which finds that arsenic pollution in groundwater is caused by the indiscriminate use of chemicals in agriculture, challenges the conclusion reached by other parties that it is basically geologic in nature. However, despite valid concerns over arsenic contamination, scare mongering by certain interests as well by the media is unwarranted.
1. Hydrogeological investigation of ground water
Sikdar and Banerjee eminent Indian Geo-Hydrologists (Journal of Human settlement, 2003) based their study on six lithostratigraphic drillings they made in North 24 Parganas, Hooghly and Murshidabad districts. Five of these were in areas that have a high concentration of arsenic. The sixth was a control block in a non-arsenious zone in North 24 Parganas. They found six heavy mineral sites in sedimentary rocks mainly belonging to the Bihar plateau, with a portion originating in the sedimentary segments of the Himalayan region. "XRD analysis reveals that illite is the dominant clay mineral in the clay/silty clay partings. No arsenic bearing mineral phase could be identified in the clay or in the sands in the arsenious zone. The concentration of arsenic in sediments generally decreases with depth and arsenic has high positive correlation with iron, manganese, copper and lead and low correlation with zinc based on multiple correlation analysis.
The mobility of arsenic from the sedimentary pyrite layer into the aquifers due to large-scale withdrawal of groundwater for agriculture and drinking purposes is due to the green revolution and outbreak of cholera in the 1960s in south Bengal. This followed rapid intake of O2(oxygen) within the pore spaces of the sediments and are believed to be due to the following geochemical processes.
- FeS2 + 2H2O + 5O2 ® FeSO4 + 2H2SO4 …(1)
- (Pyrite) (Ferrous sulphate) FeSO4 + O2 + 2H2SO4 2Fe (SO4)3 …(2)
- (Ferric sulphate) FeS2+7Fe2 (SO4)3+8H2O ®15FeSO4+8H2SO4 …(3)
Needless to say, the ferric ion thus released acts as a catalyst in further decomposition of pyrite. Sikdar and Banerjee doubt the validity of this geochemical explanation. Taking a cue from an unpublished paper by K S Subramaninan et al, they point out a conceptual anomaly in the physico-chemical understanding of geologists of yesteryear and also of S K Acharya, former director-general GSI.5 "First, equations (1) and (3) may proceed chemically, but equation (2) cannot proceed chemically in acid solution and can occur via microbial oxidation, possibly caused by the microorganism of the ferrobacillus-thiobacillus group. Second, under the above oxidising condition arsenic will be mostly in As+5 oxidation state, but in nature As+3 is dominant in groundwater as observed by the authors in groundwater samples of North 24 Parganas district Third, the mechanism does not take into account the physico-chemical characteristics of the groundwater samples. In general, the pH and bicarbonate values of groundwater samples in arsenic affected areas are above 7 and 500 mg/l respectively. Under these conditions, it is doubtful whether reactions (2) and (3) would proceed, and consequently, to what extent leaching of arsenic would occur," the two earth-scientists explained. The findings of Sikdar and Banerjee would demolish the myth - emanating from the School of Environmental Studies, Jadavpur University, and propagated by the media - that arsenic pollution is basically geologic. The two geoscientists concentrated their research investigations to lithostratigraphic and geochemical aspects. They say, "Most scientists postulate that arsenic pollution in the Ganga delta of Bengal basin is a natural phenomenon and the origin of arsenic is related to the geological setting of the Bengal basin caused by Holocene sea level rise and the Ganga-Brahmaputra deltaic sedimentation
But the geological origin and mechanism of transport of arsenic from the source to the sink cannot answer some field observations. This paper at first discusses briefly the geological origin and mobilisation of arsenic in groundwater and its drawback and then, to overcome the difficulty, presents an anthropogenic model of the genesis of arsenic in groundwater." This inference seeks to reject the inference by the Geological Survey of India (GSI), which in the late 1980s suggested that arsenic was "occurring in shallow sandy origin within a particular geological/geomorphological unit" (Sankar. Ray, EPW Commentary, November 15, 2003).
arsenic contamination in south Calcutta
2. Arsenic poisoning: man-made disaster
3. Arsenic in Groundwater Research and Rhetoric
(Last Modified:August 3, 2004)